Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The chemical symbol for Sulfur is S. Sulfur is abundant, multivalent, and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8.
What is the octet rule of sulfur?

The answer is true.Sulfur likes to gain two electrons. Sulfur (S) is an element in the third row of main group 6A (group 16) of the periodic table. Besides, how many electrons are in Sulfur? So for the element of SULFUR, you already know that the atomic number tells you the number of electrons. That means there are 16 electrons in a sulfur atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and six in shell three. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Sulfur is 16. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Find the Atomic Number. To find out the atomic number of sulfur, we can use the periodic table.
1 Answer
The octet rule is the understanding that most atoms seek to gain stability in their outer most energy level by filling the s and p orbitals of the highest energy level with eight electrons.
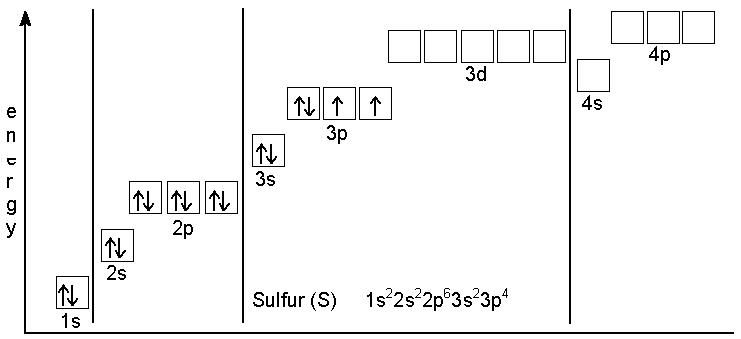
Sulfur has an electron configuration of
However, now Sulfur has 18 electrons and only 16 protons making it a -2 charge anion
I hope this was helpful.
SMARTERTEACHER
Related questions
Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO2, we can determine the hybridization of atoms.
Sulfur dioxide | SO2
Sulfur dioxide is a colourless inorganic gas and also a toxic gas. It gives a weak acid solution when dissolves in water. This gas is produced due to combustion of petroleum fuel in automobiles and industrial factories.
Lewis structure of SO2
There are two double bonds between sulfur atom and oxygen atoms in SO molecule. Also, a lone pair exists on sulfur atom and each oxygen atom has two lone pairs in SO2 lewis structure.
Hybridization of SO2
All atoms have sp2 hybridization. Each oxygen atom has one sigma bond and two lone pairs. Therefore, oxygen atoms' hybridization should be sp2. For sulfur atom, there are two sigma bonds and one lone pair to make hybridization sp2.
Simple method to determine the hybridization of atoms in covalent compounds
Apply VSEPR theory - Steps of drawing lewis structure of SO2
Following steps are used to draw the lewis structure of SO2. Each step is explained in detail in next sections. If you are a beginner to lewis structure drawing, follow these sections slowly and properly to understand it completely. Look the figures to understand each step.
- Find total number of electrons of the valance shells of sulfur and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds until most stable structure is obtained.
Total number of electrons of the valance shells of ethene
Both sulfur and oxygen belongs to the group VIA elements series. Therefore, they have six electrons in their valence shell. To find number of valence electron, these valence electrons of each element should be multiplied with their respective number of atoms in the molecule. Below, That step are done.
- Total valence electrons given by two oxygen atoms = 6 * 2 = 12
- Total valence electrons given by sulfur atom = 6 * 1 = 6
There are no charges in SO2 molecule. Therefore, no addition or reduction of valence electrons due to charges.
- Total valence electrons = 12 + 6 = 18
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, SO2 molecule, Total number of pairs of electrons are 9.
Center atom and sketch of ethene molecule
There are some requirements to be the center atom. Having a high valence is a main requirement to be a center atom. For SO2 molecule, sulfur has the highest valence than and oxygen.
Mark lone pairs on atoms
After drawing the sketch, we should start to mark lone pairs on atoms. In the drawn sketch, there are two bonds. between atoms.
- There are already two S-O bonds in the above sketch. Now, there are only seven (9-2 = 7) valence electrons pairs are remaining to draw (as mark lone pairs) the rest of the structure.
- First, mark remaining valence electrons pair as a lone pairs on oxygen atoms (outside atoms). Six valence electron pairs are marked on two oxygen atoms. Now, there are still one valence electron pair is remaining. That is marked on sulfur atom as the lone pair.
- Now, all valence electron pairs are marked as bonds and lone pairs in the sketch.
Charges on atoms
Charges on atoms are important to find the most stable structure. Therefore, we need to find the most stable structure to obtain lewis structure. Therefore, we should try to find charges if there are.
After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atoms will get a -1 charge and sulfur atom get a +2 charge. Because SO2 is a neutral molecule, overall charge of the molecule should be zero. The overall charge of the molecule is, (-1) * 2 + (+2) = 0.
Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible. In thee above structure, there are charges on oxygen atoms and sulfur atom. Now, we are going to reduce charges on these atoms as below.
- Now, we should try to minimize charges by converting a lone pair or pairs to a bond. So convert a lone pair on a oxygen atom to make a new S-O bond with sulfur atom as the following figure.
- Now there is a double bond between one oxygen atom and sulfur atom. You can see, charges are reduced now in the new structure.
- Because, there are still charges on atoms, we can try to convert a lone pair to a bond. So, convert a lone pair on other oxygen atom to make a bond with sulfur atom. With that, there are no charges on sulfur and oxygen atoms.
- Therfore, that structure should be the lewis structure of SO2
Questions
How many lone pairs sulfur has in SO2
There is only one lone pair on valence shell of sulfur atom.
Sulfur Number Of Electron Shells
SO2 lewis structure lone pairs
There are lone pairs on all atoms in SO2. Sulfur atom has one lone pair and each oxygen atom has two lone pairs. Therefore, there are total of five lone pairs on last shells of each atom in SO2.

What are the other similar lewis structures of SO2?
If we consider the shape of SO2, water, nitrogen dioxide, hydrogen sulfide, ozone have similar shape, bent. Also, if number of sigma bonds are is considered, water, nitrogen dioxide, hydrogen sulfide, sulfur dioxide all have two sigma bonds. Both SO2 and ozone has one lone pair on thei center atom.
Are there similarities in SO2 and NO2 lewis structures?
Actually, this question is weird one. Rather than discussing similarities, it's good to discuss their differences. In NO2 lewis structure, there is an unpaired electron on nitrogen atom.
Sulfur Number Of Electrons In Outer Shell
What are the similarities and differences in SO2 and SO3 lewis structures?
- Around sulfur atom, summation of lone pair and sigma bonds is three in both SO2 and SO3. Therefore, sulfur atom has sp2 hybridization.
- But, shapes around sulfur atom in SO2 and SO3 are different. In SO2, shape around sulfur atom is bent. But in SO3, shape is trigonal planar.
Sulfur Total Number Of Electrons

Related lewis structures
P2O5 lewis structureOH- lewis structureAmmonium ion (NH4+) lewis structureH2CO3 lewis structure